PRODUCTS
Centroid®
Wearable Patient Orientation, Activity, and Respiration Rate Sensor
Designed to help clinicians adhere to turn protocols and prevent pressure injuries, Centroid pairs with Root® to wirelessly monitor and display patient position and orientation, activity, and respiration rate, helping keep patients safe during extended hospital stays.
Assess Risk of Tissue Stress
Alerts for Potential Falls
Wireless & Wearable
Make Pressure Injuries a
“Never Event”
Hospital-acquired pressure injuries, or HAPIs, are categorized by the Centers for Medicare & Medicaid Services (CMS) as “never events,” yet they affect nearly 2.5 million patients annually—leading to approximately 60,000 deaths as a direct result. These preventable injuries cost U.S. hospitals an estimated $11 billion each year in treatment.1-2
~2.5 million
patients are affected by pressure injuries annually2
Cela peut être compliqué pour les hôpitaux surchargés de faire respecter les protocoles de changement de position.
Grâce à Centroid, la surveillance des patients présentant un risque de stress tissulaire est simplifiée.
Insights in Motion
Centroid monitors and tracks patient position and activity to help care teams assess the risk of tissue stress and adhere to turn protocols.
- Features trended information about past position and duration, helping clinicians identify safe and high-risk zones for repositioning
- Alerts clinicians to sudden movements, including potential falls
- Detects chest movement to provide respiration rate measurements for better-informed care
Centroid pairs with the Root® platform via Bluetooth®, providing clinicians with multiple visual ways to assess the risk of tissue stress, track patient position, and support more-informed care decisions.
- Sends patient data to the EMR
- Provides remote notifications when intervention may be needed
- Enables clinicians to monitor up to 80 Centroid-equipped patients at a glance using Masimo Patient SafetyNet™*
Centroid Details & Features
Diverse Data at Your Fingertips
- Wearable sensor identifies whether a patient is lying down, standing, sitting upright, walking, or has fallen.
- Customizable position and posture thresholds are designed to meet each hospital’s standard-of-care turn-angle goals.
- Color-coded markers (low risk, moderate risk, and high risk) help identify the amount of time a patient has spent on each part of their body, also taking into account patient self-turns.
- Customizable alarm zones help avoid patient positions that could negatively impact recovery time.
- Notifications about sudden changes in position can provide early warning of potential falls, including audible alarms on Root.
- Robust analytics and reporting capabilities support continuous improvements and compliance with hospital protocols.
Intuitive Display
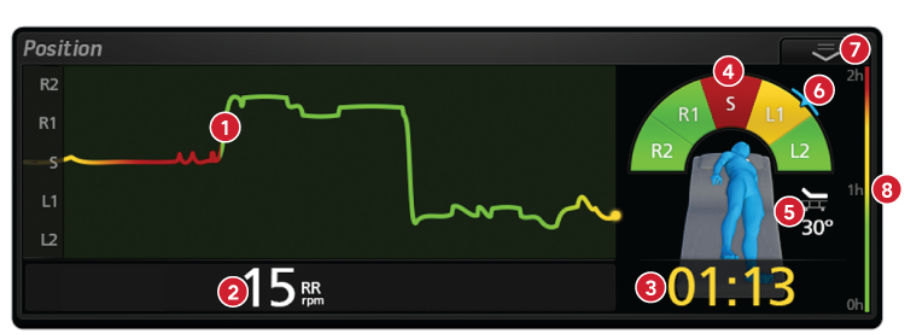
- Trend Line displays the duration of time spent in the right-side, supine, or left-side position, tracks movement, and uses color to indicate tissue stress over time.
- Respiration Rate is displayed as respirations per minute.
- Configurable Timer displays the amount of time the patient has spent in or has remaining in their current position; digit color indicates the potential severity of tissue stress.
- Time in Position Color Chart gives an aggregate view of the patient’s time in position, using color to distinguish areas that may be at high tissue stress.
- Head of Bed Angle displays the patient’s angle to the nearest degree.
- Current Patient Position is identified in blue and is displayed as a marker on the body position arch and also in a three-dimensional representation of the patient’s body.
- Action Menu provides easy access to settings.
- Time in Position Color Key uses color to correlate the length of time spent in the current position and the potential severity of tissue stress based on hospital protocol.

Diseño cómodo y portátil
- Diseñado ergonómicamente con material flexible y ligero para la comodidad del paciente
- La fijación adhesiva admite el uso continuo durante las actividades diarias
- Fabricado sin látex de caucho natural
- Batería con 4 días de vida y con la capacidad de quitar y volver a poner el sensor
RESSOURCES
Vous avez besoin de plus d’informations ? Vous trouverez ci-dessous des ressources clés sur Centroid.
Schedule an Evaluation
See how Centroid can make a difference in your healthcare organization. Please fill out the following information and a Masimo representative will be in touch with you.
References
Agency for Healthcare Research and Quality. Preventing Pressure Ulcers in Hospitals. https:// www.ahrq.gov/patient-safety/settings/hospital/resource/pressureulcer/tool/pu1.html.
* The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium. For professional use.
See instructions for use for full prescribing information including indications, contraindications, warnings and precautions. Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.
PLCO-007735/PLM-12506C-0625